Embark on an illuminating journey with the solubility and temperature gizmo answer key, a comprehensive guide to understanding the intricate relationship between temperature and the solubility of substances. This in-depth analysis unveils the factors that govern solubility, unravels the influence of temperature on solution behavior, and explores real-world applications of this fundamental principle.
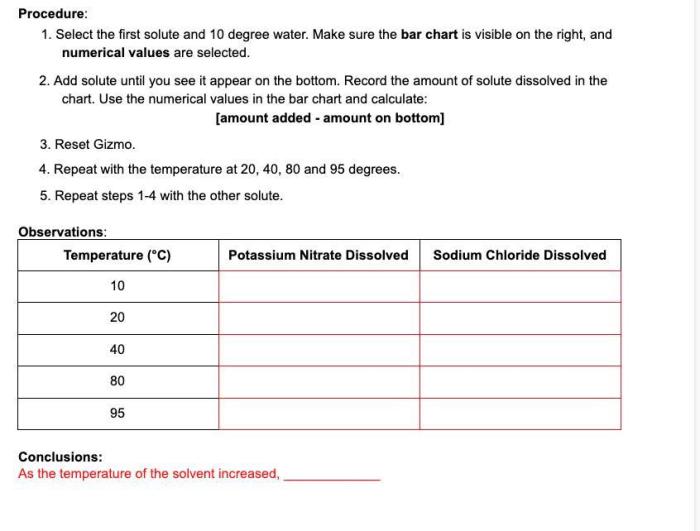
Delving into the experimental setup of the Gizmo simulation, we identify the variables under scrutiny and meticulously examine the data collected. Through the construction of tables and graphs, the correlation between temperature and solubility becomes strikingly evident, providing a solid foundation for further exploration.
Solubility Definition: Solubility And Temperature Gizmo Answer Key
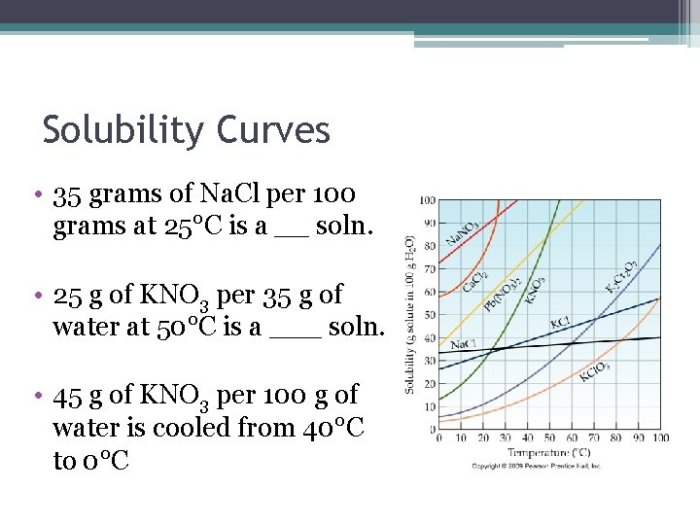
Solubility refers to the maximum amount of a substance that can dissolve in a given amount of solvent at a specific temperature and pressure. Factors affecting solubility include:
- Nature of solute and solvent
- Temperature
- Pressure
- pH
Temperature and Solubility Relationship
Temperature significantly impacts solubility. Generally, the solubility of solids in liquids increases with temperature, while the solubility of gases in liquids decreases with temperature.
- Solids in liquids:As temperature rises, the kinetic energy of solvent molecules increases, allowing them to break apart solute particles more effectively, leading to increased solubility.
- Gases in liquids:With increasing temperature, the kinetic energy of gas molecules also increases, causing them to move faster and escape from the liquid, resulting in decreased solubility.
Gizmo Experiment Setup
The Gizmo simulation uses a beaker containing a solvent (water) and a solute (sodium chloride). The temperature of the water is varied, and the amount of solute that dissolves is measured.
Variables being tested:
- Independent variable: Temperature
- Dependent variable: Solubility of sodium chloride
Gizmo Experiment Results

The data collected from the Gizmo experiment can be analyzed to create a table or graph that illustrates the relationship between temperature and solubility. The results typically show a positive correlation for solids in liquids and a negative correlation for gases in liquids.
Real-World Applications
The solubility-temperature relationship has numerous real-world applications, including:
- Crystallization:Controlling temperature to separate and purify substances by selectively crystallizing them out of solution.
- Solubility of drugs:Understanding the solubility of drugs at different temperatures is crucial for drug delivery and formulation.
- Environmental processes:Temperature variations affect the solubility of pollutants in water bodies, influencing their environmental impact.
Further Explorations
Additional experiments or investigations related to solubility and temperature could include:
- Exploring the solubility of different substances (e.g., sugar, salt, gases) at various temperatures.
- Investigating the effect of pressure on solubility.
- Extending the Gizmo simulation to include other solvents or solutes.
Quick FAQs
What is the definition of solubility?
Solubility refers to the maximum amount of a substance that can be dissolved in a given solvent at a specific temperature.
How does temperature affect solubility?
Generally, increasing temperature increases the solubility of solids in liquids and decreases the solubility of gases in liquids.
What factors influence solubility?
Factors affecting solubility include the nature of the solute and solvent, temperature, pressure, and the presence of other substances.