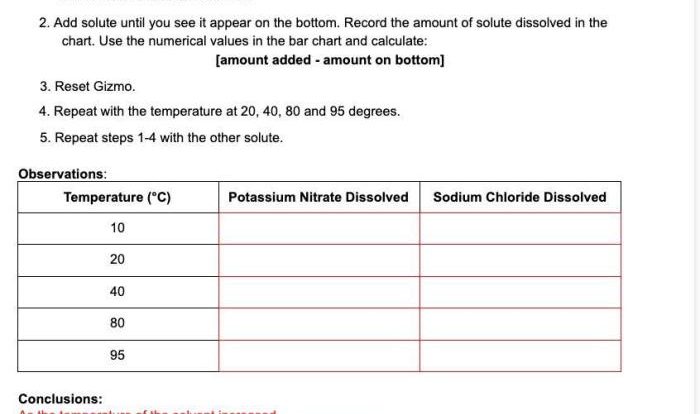
The ACS Gen Chem Formula Sheet is an indispensable tool for chemistry students, providing a comprehensive collection of formulas and information to support their studies and problem-solving. This essential resource covers a wide range of topics, from inorganic and organic chemistry to physical chemistry and electrochemistry, making it an invaluable aid for understanding and mastering the subject.
Whether you’re preparing for exams, tackling complex chemistry problems, or simply seeking a deeper understanding of chemical concepts, the ACS Gen Chem Formula Sheet is an invaluable resource that will empower you to excel in your chemistry endeavors.
General Overview of ACS Gen Chem Formula Sheet
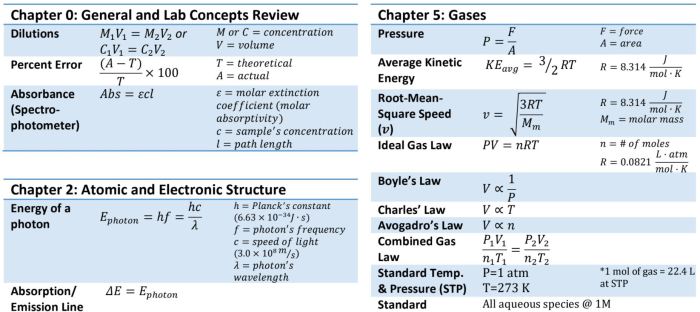
The ACS Gen Chem Formula Sheet is an essential tool for students taking general chemistry. It provides a concise summary of the most important formulas, constants, and other information needed to succeed in the course. The formula sheet is organized into several sections, including:
- Basic constants:This section includes the values of fundamental constants such as the Avogadro constant, the Faraday constant, and the gas constant.
- Thermochemistry:This section includes formulas for calculating enthalpy, entropy, and Gibbs free energy.
- Kinetics:This section includes formulas for calculating reaction rates and half-lives.
- Equilibrium:This section includes formulas for calculating equilibrium constants and concentrations.
- Acids and bases:This section includes formulas for calculating pH, pKa, and pKb.
- Electrochemistry:This section includes formulas for calculating cell potentials and electrode potentials.
- Quantum mechanics:This section includes formulas for calculating the energy levels of atoms and molecules.
The ACS Gen Chem Formula Sheet is a valuable resource for students taking general chemistry. It provides a quick and easy way to access the information needed to solve problems and understand the concepts covered in the course.
Inorganic Chemistry

Inorganic chemistry delves into the study of elements and their compounds, excluding organic molecules. It encompasses a wide range of topics, including acid-base equilibria, solubility rules, and oxidation-reduction reactions. These concepts play a crucial role in understanding the behavior of inorganic substances in various chemical systems.
Acid-Base Equilibria
Acid-base equilibria involve reactions between acids and bases, resulting in the formation of salts and water. The strength of an acid or base is determined by its ability to donate or accept protons (H+ ions). The pH of a solution, a measure of its acidity or basicity, is calculated using the following equation:“`pH =
log[H+]
When studying for the ACS General Chemistry exam, it’s essential to have a comprehensive formula sheet. This resource can be a lifesaver, providing quick access to the formulas you need. For those who are interested in legal matters, the Howard v.
Kunto case brief is also a valuable resource. However, it’s important to remember that while these resources can be helpful, they should not be relied upon as the sole source of information for either chemistry or law.
“`where [H+] represents the molar concentration of hydrogen ions in the solution.
Solubility Rules
Solubility rules predict the solubility of ionic compounds in water. These rules help determine whether a compound will dissolve, precipitate, or form a gas when added to water. Some common solubility rules include:
- Most alkali metal (Group 1) and ammonium (NH4+) salts are soluble in water.
- Most chloride (Cl-), bromide (Br-), and iodide (I-) salts are soluble in water.
- Most sulfate (SO42-) salts are soluble in water, except for those of barium (Ba2+), strontium (Sr2+), and lead (Pb2+).
Oxidation-Reduction Reactions
Oxidation-reduction reactions involve the transfer of electrons between atoms or ions. In these reactions, one species is oxidized (loses electrons) while another species is reduced (gains electrons). The oxidation state of an element is a measure of its electron gain or loss.The
half-reaction method is commonly used to balance oxidation-reduction reactions. In this method, the reaction is divided into two half-reactions, one for oxidation and one for reduction. The half-reactions are then balanced separately before combining them to form the overall balanced equation.
Organic Chemistry
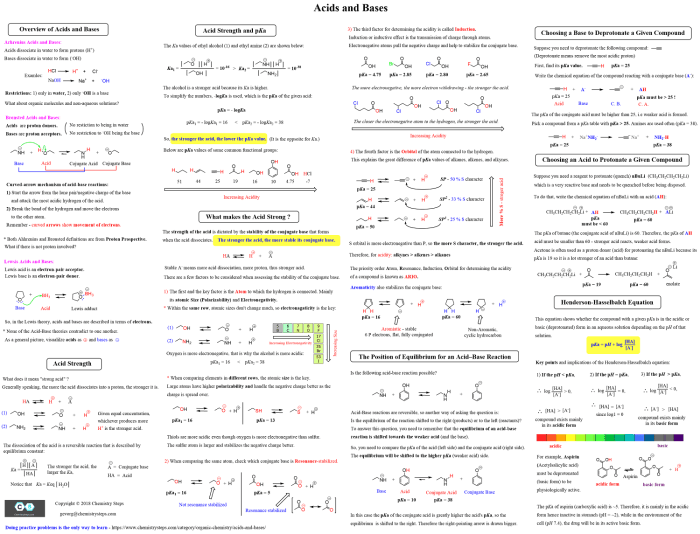
Organic chemistry is the study of compounds that contain carbon. These compounds are found in all living things and are essential for life. Organic chemistry is a vast and complex field, but some of the most important concepts include functional groups, IUPAC nomenclature, and stereochemistry.
Functional Groups
Functional groups are atoms or groups of atoms that give organic compounds their characteristic properties. Some of the most common functional groups include:
- Alkanes: Contain only carbon and hydrogen atoms, and have the general formula CnH2n+2.
- Alkenes: Contain at least one carbon-carbon double bond, and have the general formula CnH2n.
- Alkynes: Contain at least one carbon-carbon triple bond, and have the general formula CnH2n-2.
- Alcohols: Contain a hydroxyl group (-OH), and have the general formula CnH2n+1OH.
- Ethers: Contain an ether group (-O-), and have the general formula CnH2n+1OR.
- Aldehydes: Contain a carbonyl group (-C=O), and have the general formula CnH2n+1CHO.
- Ketones: Contain a carbonyl group (-C=O), and have the general formula CnH2n+1COR.
- Carboxylic acids: Contain a carboxyl group (-COOH), and have the general formula CnH2n+1COOH.
- Amines: Contain a nitrogen atom with one or more hydrogen atoms attached, and have the general formula CnH2n+1NH2.
IUPAC Nomenclature, Acs gen chem formula sheet
IUPAC nomenclature is a system for naming organic compounds. It is based on the structure of the compound and the functional groups that it contains. IUPAC nomenclature is used by chemists all over the world to ensure that everyone is using the same names for the same compounds.
Stereochemistry
Stereochemistry is the study of the three-dimensional structure of molecules. It is important in organic chemistry because the three-dimensional structure of a molecule can affect its properties. For example, the optical activity of a molecule is determined by its stereochemistry.
Physical Chemistry

Physical chemistry is the study of the physical and chemical properties of matter, with a focus on the relationship between energy and matter. It deals with macroscopic and microscopic phenomena, and its principles are used in various fields, including chemical engineering, materials science, and biochemistry.
Key formulas in physical chemistry include:
Thermodynamics
- First Law of Thermodynamics:ΔU = Q – W, where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
- Second Law of Thermodynamics:The entropy of an isolated system can never decrease over time.
- Gibbs Free Energy:G = H – TS, where G is the Gibbs free energy, H is the enthalpy, T is the temperature, and S is the entropy.
Kinetics
- Rate Law:Rate = k[A]^m[B]^n, where Rate is the rate of reaction, k is the rate constant, [A] and [B] are the concentrations of reactants A and B, and m and n are the orders of reaction with respect to A and B, respectively.
- Arrhenius Equation:k = Ae^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature.
- Half-Life:t 1/2= (ln 2)/k, where t 1/2is the half-life, and k is the rate constant.
Electrochemistry
- Nernst Equation:E = E° – (RT/nF)lnQ, where E is the cell potential, E° is the standard cell potential, R is the gas constant, T is the temperature, n is the number of electrons transferred, F is the Faraday constant, and Q is the reaction quotient.
- Faraday’s Law of Electrolysis:m = (ItM)/nF, where m is the mass of substance deposited or evolved, I is the current, t is the time, M is the molar mass of the substance, n is the number of electrons transferred, and F is the Faraday constant.
- Ohm’s Law:V = IR, where V is the voltage, I is the current, and R is the resistance.
Applications and Usage
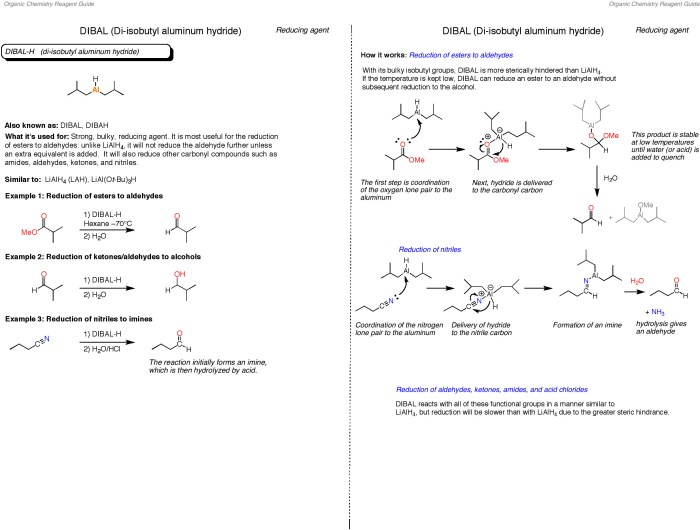
The ACS Gen Chem Formula Sheet is a valuable resource for students and professionals in various chemistry settings. Its concise and organized presentation of essential formulas and concepts makes it an indispensable tool for:
Problem-solving
The formula sheet provides a quick and easy reference for students when solving chemistry problems. It eliminates the need to search through textbooks or notes for specific formulas, saving time and reducing the likelihood of errors.
Exam preparation
The formula sheet is an invaluable aid during exam preparation. It allows students to focus their studies on understanding the concepts behind the formulas rather than memorizing them individually. By having the formulas readily available, students can allocate more time to practicing problem-solving and developing their critical thinking skills.
Laboratory work
In the laboratory, the formula sheet serves as a handy reference for essential equations and constants. It helps students quickly access the necessary information without interrupting their experimental work. Additionally, it can assist in troubleshooting experimental procedures and ensuring accurate data analysis.
Limitations and Considerations
The ACS Gen Chem Formula Sheet is a valuable resource, but it has some limitations and considerations that users should be aware of.One limitation is that the formula sheet does not provide detailed explanations or derivations of the formulas. It assumes that users have a basic understanding of the underlying concepts and principles behind the formulas.
Without this understanding, users may not be able to apply the formulas correctly or troubleshoot problems.Another consideration is that the formula sheet is not a substitute for a comprehensive understanding of general chemistry. It is a tool that can be used to supplement learning and review, but it should not be relied upon as the sole source of information.
Students should still attend lectures, read textbooks, and practice solving problems to develop a deep understanding of the subject matter.
Importance of Understanding Underlying Concepts
It is crucial to understand the underlying concepts behind the formulas on the ACS Gen Chem Formula Sheet. This understanding allows users to:
- Apply the formulas correctly in different contexts.
- Troubleshoot problems and identify errors in calculations.
- Extend their knowledge beyond the formulas themselves and develop a deeper understanding of general chemistry.
Without a solid grasp of the underlying concepts, users may be limited in their ability to use the formula sheet effectively and may encounter difficulties in applying the formulas to real-world problems.
FAQ Guide: Acs Gen Chem Formula Sheet
What is the purpose of the ACS Gen Chem Formula Sheet?
The ACS Gen Chem Formula Sheet provides a comprehensive collection of formulas and information to support students in their chemistry studies and problem-solving.
What topics does the ACS Gen Chem Formula Sheet cover?
The ACS Gen Chem Formula Sheet covers a wide range of topics, including inorganic chemistry, organic chemistry, physical chemistry, and electrochemistry.
How can I use the ACS Gen Chem Formula Sheet?
The ACS Gen Chem Formula Sheet can be used for problem-solving, exam preparation, laboratory work, and to deepen your understanding of chemical concepts.